Visible light" redirects here. For light that cannot be seen with human eye, see
Electromagnetic radiation. For other uses, see
Light (disambiguation) and
Visible light (disambiguation).
A triangular
prism dispersing a beam of white light. The longer wavelengths (red) and the shorter wavelengths (blue) are separated.
| Modern physics |
|---|


|
|
|
|
|
|
|
|
|
|
|
Light is
electromagnetic radiation within a certain portion of the
electromagnetic spectrum. The word usually refers to
visible light, which is the
visible spectrum that is visible to the
human eye and is responsible for the sense of
sight.
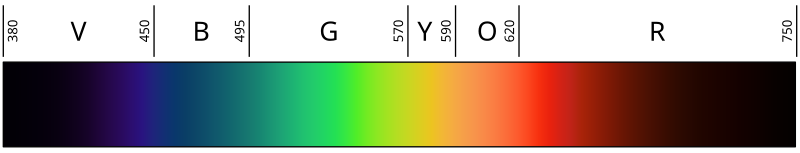
[1] Visible light is usually defined as having
wavelengths in the range of 400–700
nanometres (nm), or 4.00 × 10
−7 to 7.00 × 10
−7 m, between the
infrared (with longer wavelengths) and the
ultraviolet (with shorter wavelengths).
[2][3] This wavelength means a
frequency range of roughly 430–750
terahertz (THz).
Beam of sun light inside the cavity of Rocca ill'Abissu at
Fondachelli Fantina, Sicily
The main source of light on Earth is the
Sun.
Sunlight provides the
energy that
green plants use to create
sugars mostly in the form of
starches, which release energy into the living things that digest them. This process of
photosynthesis provides virtually all the energy used by living things. Historically, another important source of light for humans has been
fire, from ancient campfires to modern
kerosene lamps. With the development of
electric lights and
power systems, electric lighting has effectively replaced firelight. Some species of animals generate their own light, a process called
bioluminescence. For example,
fireflies use light to locate mates, and
vampire squids use it to hide themselves from prey.
The primary properties of visible light are
intensity, propagation direction, frequency or wavelength
spectrum, and
polarization, while its
speed in a vacuum, 299,792,458 metres per second, is one of the fundamental
constants
of nature. Visible light, as with all types of electromagnetic
radiation (EMR), is experimentally found to always move at this speed in
a vacuum.
[4]
In
physics, the term
light sometimes refers to electromagnetic radiation of any wavelength, whether visible or not.
[5][6] In this sense,
gamma rays,
X-rays,
microwaves and
radio waves
are also light. Like all types of EM radiation, visible light
propagates as waves. However, the energy imparted by the waves is
absorbed at single locations the way particles are absorbed. The
absorbed energy of the EM waves is called a photon, and represents the
quanta of light. When a wave of light is transformed and absorbed as a
photon, the energy of the wave instantly collapses to a single location,
and this location is where the photon "arrives." This is what is called
the
wave function collapse. This dual wave-like and particle-like nature of light is known as the
wave–particle duality. The study of light, known as
optics, is an important research area in modern physics.
Electromagnetic spectrum and visible light
Generally, EM radiation (the designation "radiation" excludes static electric, magnetic, and
near fields), or EMR, is classified by wavelength into
radio waves,
microwaves,
infrared, the
visible spectrum that we perceive as light,
ultraviolet,
X-rays, and
gamma rays.
The behavior of EMR depends on its wavelength. Higher frequencies
have shorter wavelengths, and lower frequencies have longer
wavelengths. When EMR interacts with single atoms and molecules, its
behavior depends on the amount of energy per quantum it carries.
EMR in the visible light region consists of quanta (called
photons)
that are at the lower end of the energies that are capable of causing
electronic excitation within molecules, which leads to changes in the
bonding or chemistry of the molecule. At the lower end of the visible
light spectrum, EMR becomes invisible to humans (infrared) because its
photons no longer have enough individual energy to cause a lasting
molecular change (a change in conformation) in the visual molecule
retinal in the human retina, which change triggers the sensation of vision.
There exist animals that are sensitive to various types of infrared, but not by means of quantum-absorption.
Infrared sensing in snakes depends on a kind of natural
thermal imaging,
in which tiny packets of cellular water are raised in temperature by
the infrared radiation. EMR in this range causes molecular vibration and
heating effects, which is how these animals detect it.
Above the range of visible light, ultraviolet light becomes
invisible to humans, mostly because it is absorbed by the cornea below
360
nm and the internal lens below 400 nm. Furthermore, the
rods and
cones located in the
retina
of the human eye cannot detect the very short (below 360 nm)
ultraviolet wavelengths and are in fact damaged by ultraviolet. Many
animals with eyes that do not require lenses (such as insects and
shrimp) are able to detect ultraviolet, by quantum photon-absorption
mechanisms, in much the same chemical way that humans detect visible
light.
Various sources define visible light as narrowly as 420–680 nm
[7][8] to as broadly as 380–800 nm.
[9][10] Under ideal laboratory conditions, people can see infrared up to at least 1050 nm;
[11] children and young adults may perceive ultraviolet wavelengths down to about 310–313 nm.
[12][13][14]
Plant growth is also affected by the color spectrum of light, a process known as
photomorphogenesis.
Speed of light
The speed of light in a
vacuum is defined to be exactly 299,792,458
m/s
(approx. 186,282 miles per second). The fixed value of the speed of
light in SI units results from the fact that the metre is now defined in
terms of the speed of light. All forms of electromagnetic radiation
move at exactly this same speed in vacuum.
Different
physicists have attempted to measure the speed of light throughout history.
Galileo
attempted to measure the speed of light in the seventeenth century. An
early experiment to measure the speed of light was conducted by
Ole Rømer, a Danish physicist, in 1676. Using a
telescope, Rømer observed the motions of
Jupiter and one of its
moons,
Io.
Noting discrepancies in the apparent period of Io's orbit, he
calculated that light takes about 22 minutes to traverse the diameter of
Earth's orbit.
[15]
However, its size was not known at that time. If Rømer had known the
diameter of the Earth's orbit, he would have calculated a speed of
227,000,000 m/s.
Another more accurate measurement of the speed of light was performed in Europe by
Hippolyte Fizeau in 1849. Fizeau directed a beam of light at a mirror several kilometers away. A rotating
cog wheel
was placed in the path of the light beam as it traveled from the
source, to the mirror and then returned to its origin. Fizeau found
that at a certain rate of rotation, the beam would pass through one gap
in the wheel on the way out and the next gap on the way back. Knowing
the distance to the mirror, the number of teeth on the wheel, and the
rate of rotation, Fizeau was able to calculate the speed of light as
313,000,000 m/s.
Léon Foucault carried out an experiment which used rotating mirrors to obtain a value of 298,000,000 m/s in 1862.
Albert A. Michelson
conducted experiments on the speed of light from 1877 until his death
in 1931. He refined Foucault's methods in 1926 using improved rotating
mirrors to measure the time it took light to make a round trip from
Mount Wilson to
Mount San Antonio in California. The precise measurements yielded a speed of 299,796,000 m/s.
[16]
The effective velocity of light in various transparent substances containing ordinary
matter, is less than in vacuum. For example, the speed of light in water is about 3/4 of that in vacuum.
Two independent teams of physicists were said to bring light to a "complete standstill" by passing it through a
Bose–Einstein condensate of the element
rubidium, one team at
Harvard University and the
Rowland Institute for Science in Cambridge, Massachusetts, and the other at the
Harvard–Smithsonian Center for Astrophysics, also in Cambridge.
[17]
However, the popular description of light being "stopped" in these
experiments refers only to light being stored in the excited states of
atoms, then re-emitted at an arbitrary later time, as stimulated by a
second laser pulse. During the time it had "stopped" it had ceased to be
light.
Optics
The study of light and the interaction of light and
matter is termed
optics. The observation and study of
optical phenomena such as
rainbows and the
aurora borealis offer many clues as to the nature of light.
Refraction
An example of refraction of light. The straw appears bent, because of refraction of light as it enters liquid from air.
A cloud illuminated by sunlight
Refraction is the bending of light rays when passing through a
surface between one transparent material and another. It is described by
Snell's Law:

where θ
1 is the angle between the ray and the
surface normal in the first medium, θ
2 is the angle between the ray and the surface normal in the second medium, and n
1 and n
2 are the
indices of refraction,
n = 1 in a
vacuum and
n > 1 in a
transparent substance.
When a beam of light crosses the boundary between a vacuum and
another medium, or between two different media, the wavelength of the
light changes, but the frequency remains constant. If the beam of light
is not
orthogonal
(or rather normal) to the boundary, the change in wavelength results in
a change in the direction of the beam. This change of direction is
known as
refraction.
The refractive quality of
lenses is frequently used to manipulate light in order to change the apparent size of images.
Magnifying glasses,
spectacles,
contact lenses,
microscopes and
refracting telescopes are all examples of this manipulation.
Light sources
There are many sources of light. A body at a given temperature emits a characteristic spectrum of
black-body radiation. A simple thermal source is sunlight, the radiation emitted by the
chromosphere of the
Sun
at around 6,000 kelvins (5,730 degrees Celsius; 10,340 degrees
Fahrenheit) peaks in the visible region of the electromagnetic spectrum
when plotted in wavelength units
[18] and roughly 44% of sunlight energy that reaches the ground is visible.
[19] Another example is
incandescent light bulbs,
which emit only around 10% of their energy as visible light and the
remainder as infrared. A common thermal light source in history is the
glowing solid particles in
flames, but these also emit most of their radiation in the infrared, and only a fraction in the visible spectrum.
The peak of the blackbody spectrum is in the deep infrared, at about 10
micrometre
wavelength, for relatively cool objects like human beings. As the
temperature increases, the peak shifts to shorter wavelengths, producing
first a red glow, then a white one, and finally a blue-white colour as
the peak moves out of the visible part of the spectrum and into the
ultraviolet. These colours can be seen when metal is heated to "red hot"
or "white hot". Blue-white
thermal emission is not often seen, except in stars (the commonly seen pure-blue colour in a
gas flame or a
welder's
torch is in fact due to molecular emission, notably by CH radicals
(emitting a wavelength band around 425 nm, and is not seen in stars or
pure thermal radiation).
Atoms emit and absorb light at characteristic energies. This produces "
emission lines" in the spectrum of each atom.
Emission can be
spontaneous, as in
light-emitting diodes,
gas discharge lamps (such as
neon lamps and
neon signs,
mercury-vapor lamps, etc.), and flames (light from the hot gas itself—so, for example,
sodium in a gas flame emits characteristic yellow light). Emission can also be
stimulated, as in a
laser or a microwave
maser.
Deceleration of a free charged particle, such as an
electron, can produce visible radiation:
cyclotron radiation,
synchrotron radiation, and
bremsstrahlung
radiation are all examples of this. Particles moving through a medium
faster than the speed of light in that medium can produce visible
Cherenkov radiation. Certain chemicals produce visible radiation by
chemoluminescence. In living things, this process is called
bioluminescence. For example,
fireflies produce light by this means, and boats moving through water can disturb plankton which produce a glowing wake.
Certain substances produce light when they are illuminated by more energetic radiation, a process known as
fluorescence. Some substances emit light slowly after excitation by more energetic radiation. This is known as
phosphorescence. Phosphorescent materials can also be excited by bombarding them with subatomic particles.
Cathodoluminescence is one example. This mechanism is used in
cathode ray tube television sets and
computer monitors.
Certain other mechanisms can produce light:
When the concept of light is intended to include very-high-energy
photons (gamma rays), additional generation mechanisms include:
Units and measures
Light is measured with two main alternative sets of units:
radiometry consists of measurements of light power at all wavelengths, while
photometry
measures light with wavelength weighted with respect to a standardised
model of human brightness perception. Photometry is useful, for
example, to quantify
Illumination (lighting) intended for human use. The SI units for both systems are summarised in the following tables.
Table 1. SI radiometry units
| Quantity
|
Unit
|
Dimension
|
Notes
|
| Name
|
Symbol[nb 1]
|
Name
|
Symbol
|
Symbol
|
| Radiant energy
|
Qe[nb 2]
|
joule
|
J
|
M⋅L2⋅T−2
|
Energy of electromagnetic radiation.
|
| Radiant energy density
|
we
|
joule per cubic metre
|
J/m3
|
M⋅L−1⋅T−2
|
Radiant energy per unit volume.
|
| Radiant flux
|
Φe[nb 2]
|
watt
|
W = J/s
|
M⋅L2⋅T−3
|
Radiant energy emitted, reflected, transmitted or received, per unit time. This is sometimes also called "radiant power".
|
| Spectral flux
|
Φe,ν[nb 3]
or
Φe,λ[nb 4]
|
watt per hertz
or
watt per metre
|
W/Hz
or
W/m
|
M⋅L2⋅T−2
or
M⋅L⋅T−3
|
Radiant flux per unit frequency or wavelength. The latter is commonly measured in W⋅nm−1.
|
| Radiant intensity
|
Ie,Ω[nb 5]
|
watt per steradian
|
W/sr
|
M⋅L2⋅T−3
|
Radiant flux emitted, reflected, transmitted or received, per unit solid angle. This is a directional quantity.
|
| Spectral intensity
|
Ie,Ω,ν[nb 3]
or
Ie,Ω,λ[nb 4]
|
watt per steradian per hertz
or
watt per steradian per metre
|
W⋅sr−1⋅Hz−1
or
W⋅sr−1⋅m−1
|
M⋅L2⋅T−2
or
M⋅L⋅T−3
|
Radiant intensity per unit frequency or wavelength. The latter is commonly measured in W⋅sr−1⋅nm−1. This is a directional quantity.
|
| Radiance
|
Le,Ω[nb 5]
|
watt per steradian per square metre
|
W⋅sr−1⋅m−2
|
M⋅T−3
|
Radiant flux emitted, reflected, transmitted or received by a surface, per unit solid angle per unit projected area. This is a directional quantity. This is sometimes also confusingly called "intensity".
|
| Spectral radiance
|
Le,Ω,ν[nb 3]
or
Le,Ω,λ[nb 4]
|
watt per steradian per square metre per hertz
or
watt per steradian per square metre, per metre
|
W⋅sr−1⋅m−2⋅Hz−1
or
W⋅sr−1⋅m−3
|
M⋅T−2
or
M⋅L−1⋅T−3
|
Radiance of a surface per unit frequency or wavelength. The latter is commonly measured in W⋅sr−1⋅m−2⋅nm−1. This is a directional quantity. This is sometimes also confusingly called "spectral intensity".
|
Irradiance
Flux density
|
Ee[nb 2]
|
watt per square metre
|
W/m2
|
M⋅T−3
|
Radiant flux received by a surface per unit area. This is sometimes also confusingly called "intensity".
|
Spectral irradiance
Spectral flux density
|
Ee,ν[nb 3]
or
Ee,λ[nb 4]
|
watt per square metre per hertz
or
watt per square metre, per metre
|
W⋅m−2⋅Hz−1
or
W/m3
|
M⋅T−2
or
M⋅L−1⋅T−3
|
Irradiance of a surface per unit frequency or wavelength.
This is sometimes also confusingly called "spectral intensity". Non-SI
units of spectral flux density include jansky (1 Jy = 10−26 W⋅m−2⋅Hz−1) and solar flux unit (1 sfu = 10−22 W⋅m−2⋅Hz−1 = 104 Jy).
|
| Radiosity
|
Je[nb 2]
|
watt per square metre
|
W/m2
|
M⋅T−3
|
Radiant flux leaving (emitted, reflected and transmitted by) a surface per unit area. This is sometimes also confusingly called "intensity".
|
| Spectral radiosity
|
Je,ν[nb 3]
or
Je,λ[nb 4]
|
watt per square metre per hertz
or
watt per square metre, per metre
|
W⋅m−2⋅Hz−1
or
W/m3
|
M⋅T−2
or
M⋅L−1⋅T−3
|
Radiosity of a surface per unit frequency or wavelength. The latter is commonly measured in W⋅m−2⋅nm−1. This is sometimes also confusingly called "spectral intensity".
|
| Radiant exitance
|
Me[nb 2]
|
watt per square metre
|
W/m2
|
M⋅T−3
|
Radiant flux emitted by a surface per unit area. This
is the emitted component of radiosity. "Radiant emittance" is an old
term for this quantity. This is sometimes also confusingly called
"intensity".
|
| Spectral exitance
|
Me,ν[nb 3]
or
Me,λ[nb 4]
|
watt per square metre per hertz
or
watt per square metre, per metre
|
W⋅m−2⋅Hz−1
or
W/m3
|
M⋅T−2
or
M⋅L−1⋅T−3
|
Radiant exitance of a surface per unit frequency or wavelength. The latter is commonly measured in W⋅m−2⋅nm−1. "Spectral emittance" is an old term for this quantity. This is sometimes also confusingly called "spectral intensity".
|
| Radiant exposure
|
He
|
joule per square metre
|
J/m2
|
M⋅T−2
|
Radiant energy received by a surface per unit area, or equivalently irradiance of a surface integrated over time of irradiation. This is sometimes also called "radiant fluence".
|
| Spectral exposure
|
He,ν[nb 3]
or
He,λ[nb 4]
|
joule per square metre per hertz
or
joule per square metre, per metre
|
J⋅m−2⋅Hz−1
or
J/m3
|
M⋅T−1
or
M⋅L−1⋅T−2
|
Radiant exposure of a surface per unit frequency or wavelength. The latter is commonly measured in J⋅m−2⋅nm−1. This is sometimes also called "spectral fluence".
|
| Hemispherical emissivity
|
ε
|
|
|
1
|
Radiant exitance of a surface, divided by that of a black body at the same temperature as that surface.
|
| Spectral hemispherical emissivity
|
εν
or
ελ
|
|
|
1
|
Spectral exitance of a surface, divided by that of a black body at the same temperature as that surface.
|
| Directional emissivity
|
εΩ
|
|
|
1
|
Radiance emitted by a surface, divided by that emitted by a black body at the same temperature as that surface.
|
| Spectral directional emissivity
|
εΩ,ν
or
εΩ,λ
|
|
|
1
|
Spectral radiance emitted by a surface, divided by that of a black body at the same temperature as that surface.
|
| Hemispherical absorptance
|
A
|
|
|
1
|
Radiant flux absorbed by a surface, divided by that received by that surface. This should not be confused with "absorbance".
|
| Spectral hemispherical absorptance
|
Aν
or
Aλ
|
|
|
1
|
Spectral flux absorbed by a surface, divided by that received by that surface. This should not be confused with "spectral absorbance".
|
| Directional absorptance
|
AΩ
|
|
|
1
|
Radiance absorbed by a surface, divided by the radiance incident onto that surface. This should not be confused with "absorbance".
|
| Spectral directional absorptance
|
AΩ,ν
or
AΩ,λ
|
|
|
1
|
Spectral radiance absorbed by a surface, divided by the spectral radiance incident onto that surface. This should not be confused with "spectral absorbance".
|
| Hemispherical reflectance
|
R
|
|
|
1
|
Radiant flux reflected by a surface, divided by that received by that surface.
|
| Spectral hemispherical reflectance
|
Rν
or
Rλ
|
|
|
1
|
Spectral flux reflected by a surface, divided by that received by that surface.
|
| Directional reflectance
|
RΩ
|
|
|
1
|
Radiance reflected by a surface, divided by that received by that surface.
|
| Spectral directional reflectance
|
RΩ,ν
or
RΩ,λ
|
|
|
1
|
Spectral radiance reflected by a surface, divided by that received by that surface.
|
| Hemispherical transmittance
|
T
|
|
|
1
|
Radiant flux transmitted by a surface, divided by that received by that surface.
|
| Spectral hemispherical transmittance
|
Tν
or
Tλ
|
|
|
1
|
Spectral flux transmitted by a surface, divided by that received by that surface.
|
| Directional transmittance
|
TΩ
|
|
|
1
|
Radiance transmitted by a surface, divided by that received by that surface.
|
| Spectral directional transmittance
|
TΩ,ν
or
TΩ,λ
|
|
|
1
|
Spectral radiance transmitted by a surface, divided by that received by that surface.
|
| Hemispherical attenuation coefficient
|
μ
|
reciprocal metre
|
m−1
|
L−1
|
Radiant flux absorbed and scattered by a volume per unit length, divided by that received by that volume.
|
| Spectral hemispherical attenuation coefficient
|
μν
or
μλ
|
reciprocal metre
|
m−1
|
L−1
|
Spectral radiant flux absorbed and scattered by a volume per unit length, divided by that received by that volume.
|
| Directional attenuation coefficient
|
μΩ
|
reciprocal metre
|
m−1
|
L−1
|
Radiance absorbed and scattered by a volume per unit length, divided by that received by that volume.
|
| Spectral directional attenuation coefficient
|
μΩ,ν
or
μΩ,λ
|
reciprocal metre
|
m−1
|
L−1
|
Spectral radiance absorbed and scattered by a volume per unit length, divided by that received by that volume.
|
| See also: SI · Radiometry · Photometry · (Compare)
|
The photometry units are different from most systems of physical
units in that they take into account how the human eye responds to
light. The
cone cells
in the human eye are of three types which respond differently across
the visible spectrum, and the cumulative response peaks at a wavelength
of around 555 nm. Therefore, two sources of light which produce the same
intensity (W/m
2) of visible light do not necessarily appear
equally bright. The photometry units are designed to take this into
account, and therefore are a better representation of how "bright" a
light appears to be than raw intensity. They relate to raw
power by a quantity called
luminous efficacy,
and are used for purposes like determining how to best achieve
sufficient illumination for various tasks in indoor and outdoor
settings. The illumination measured by a
photocell
sensor does not necessarily correspond to what is perceived by the
human eye, and without filters which may be costly, photocells and
charge-coupled devices (CCD) tend to respond to some
infrared,
ultraviolet or both.
Light pressure
Light exerts physical pressure on objects in its path, a phenomenon
which can be deduced by Maxwell's equations, but can be more easily
explained by the particle nature of light: photons strike and transfer
their momentum. Light pressure is equal to the power of the light beam
divided by
c, the speed of light.
Due to the magnitude of
c, the effect of light pressure is negligible for everyday objects.
For example, a one-milliwatt
laser pointer exerts a force of about 3.3
piconewtons on the object being illuminated; thus, one could lift a
U.S. penny with laser pointers, but doing so would require about 30 billion 1-mW laser pointers.
[20] However, in
nanometre-scale applications such as
nanoelectromechanical systems
(|NEMS), the effect of light pressure is more significant, and
exploiting light pressure to drive NEMS mechanisms and to flip
nanometre-scale physical switches in integrated circuits is an active
area of research.
[21] At larger scales, light pressure can cause
asteroids to spin faster,
[22] acting on their irregular shapes as on the vanes of a
windmill.
The possibility of making
solar sails that would accelerate spaceships in space is also under investigation.
[23][24]
Although the motion of the
Crookes radiometer
was originally attributed to light pressure, this interpretation is
incorrect; the characteristic Crookes rotation is the result of a
partial vacuum.
[25] This should not be confused with the
Nichols radiometer, in which the (slight) motion caused by torque (though not enough for full rotation against friction)
is directly caused by light pressure.
[26]
As a consequence of light pressure,
Einstein[27]
in 1909 predicted the existence of "radiation friction" which would
oppose the movement of matter. He wrote, "radiation will exert pressure
on both sides of the plate. The forces of pressure exerted on the two
sides are equal if the plate is at rest. However, if it is in motion,
more radiation will be reflected on the surface that is ahead during the
motion (front surface) than on the back surface. The backwardacting
force of pressure exerted on the front surface is thus larger than the
force of pressure acting on the back. Hence, as the resultant of the two
forces, there remains a force that counteracts the motion of the plate
and that increases with the velocity of the plate. We will call this
resultant 'radiation friction' in brief."
Historical theories about light, in chronological order
Classical Greece and Hellenism
In the fifth century BC,
Empedocles postulated that everything was composed of
four elements; fire, air, earth and water. He believed that
Aphrodite
made the human eye out of the four elements and that she lit the fire
in the eye which shone out from the eye making sight possible. If this
were true, then one could see during the night just as well as during
the day, so Empedocles postulated an interaction between rays from the
eyes and rays from a source such as the sun.
[28]
In about 300 BC,
Euclid wrote
Optica,
in which he studied the properties of light. Euclid postulated that
light travelled in straight lines and he described the laws of
reflection and studied them mathematically. He questioned that sight is
the result of a beam from the eye, for he asks how one sees the stars
immediately, if one closes one's eyes, then opens them at night. If the
beam from the eye travels infinitely fast this is not a problem.
[29]
In 55 BC,
Lucretius, a Roman who carried on the ideas of earlier Greek
atomists,
wrote that "The light & heat of the sun; these are composed of
minute atoms which, when they are shoved off, lose no time in shooting
right across the interspace of air in the direction imparted by the
shove." (from
On the nature of the Universe). Despite being similar to later particle theories, Lucretius's views were not generally accepted.
Ptolemy (c. 2nd century) wrote about the
refraction of light in his book
Optics.
[30]
Classical India
In
ancient India, the
Hindu schools of
Samkhya and
Vaisheshika,
from around the early centuries AD developed theories on light.
According to the Samkhya school, light is one of the five fundamental
"subtle" elements (
tanmatra) out of which emerge the gross elements. The
atomicity of these elements is not specifically mentioned and it appears that they were actually taken to be continuous.
[31]
On the other hand, the Vaisheshika school gives an
atomic theory of the physical world on the non-atomic ground of
ether, space and time. (See
Indian atomism.) The basic atoms are those of earth (
prthivi), water (
pani), fire (
agni), and air (
vayu) Light rays are taken to be a stream of high velocity of
tejas (fire) atoms. The particles of light can exhibit different characteristics depending on the speed and the arrangements of the
tejas atoms.
[citation needed]
The
Vishnu Purana refers to sunlight as "the seven rays of the sun".
[31]
The Indian
Buddhists, such as
Dignāga in the 5th century and
Dharmakirti
in the 7th century, developed a type of atomism that is a philosophy
about reality being composed of atomic entities that are momentary
flashes of light or energy. They viewed light as being an atomic entity
equivalent to energy.
[31]
Descartes
René Descartes (1596–1650) held that light was a
mechanical property of the luminous body, rejecting the "forms" of
Ibn al-Haytham and
Witelo as well as the "species" of
Bacon,
Grosseteste, and
Kepler.
[32] In 1637 he published a theory of the
refraction
of light that assumed, incorrectly, that light travelled faster in a
denser medium than in a less dense medium. Descartes arrived at this
conclusion by analogy with the behaviour of sound waves.
[citation needed]
Although Descartes was incorrect about the relative speeds, he was
correct in assuming that light behaved like a wave and in concluding
that refraction could be explained by the speed of light in different
media.
Descartes is not the first to use the mechanical analogies but
because he clearly asserts that light is only a mechanical property of
the luminous body and the transmitting medium, Descartes' theory of
light is regarded as the start of modern physical optics.
[32]
Particle theory
Pierre Gassendi (1592–1655), an atomist, proposed a particle theory of light which was published posthumously in the 1660s.
Isaac Newton studied Gassendi's work at an early age, and preferred his view to Descartes' theory of the
plenum. He stated in his
Hypothesis of Light of 1675 that light was composed of
corpuscles
(particles of matter) which were emitted in all directions from a
source. One of Newton's arguments against the wave nature of light was
that waves were known to bend around obstacles, while light travelled
only in straight lines. He did, however, explain the phenomenon of the
diffraction of light (which had been observed by
Francesco Grimaldi) by allowing that a light particle could create a localised wave in the
aether.
Newton's theory could be used to predict the
reflection of light, but could only explain
refraction by incorrectly assuming that light accelerated upon entering a denser
medium because the
gravitational pull was greater. Newton published the final version of his theory in his
Opticks of 1704. His reputation helped the
particle theory of light to hold sway during the 18th century. The particle theory of light led
Laplace
to argue that a body could be so massive that light could not escape
from it. In other words, it would become what is now called a
black hole.
Laplace withdrew his suggestion later, after a wave theory of light
became firmly established as the model for light (as has been explained,
neither a particle or wave theory is fully correct). A translation of
Newton's essay on light appears in
The large scale structure of space-time, by
Stephen Hawking and
George F. R. Ellis.
The fact that light could be
polarized was for the first time qualitatively explained by Newton using the particle theory.
Étienne-Louis Malus in 1810 created a mathematical particle theory of polarization.
Jean-Baptiste Biot
in 1812 showed that this theory explained all known phenomena of light
polarization. At that time the polarization was considered as the proof
of the particle theory.
Wave theory
To explain the origin of
colors,
Robert Hooke (1635–1703) developed a "pulse theory" and compared the spreading of light to that of waves in water in his 1665 work
Micrographia ("Observation IX"). In 1672 Hooke suggested that light's vibrations could be
perpendicular to the direction of propagation.
Christiaan Huygens (1629–1695) worked out a mathematical wave theory of light in 1678, and published it in his
Treatise on light in 1690. He proposed that light was emitted in all directions as a series of waves in a medium called the
Luminiferous ether. As waves are not affected by gravity, it was assumed that they slowed down upon entering a denser medium.
[33]
The wave theory predicted that light waves could interfere with each other like sound waves (as noted around 1800 by
Thomas Young). Young showed by means of a
diffraction experiment that light behaved as waves. He also proposed that different
colours were caused by different
wavelengths
of light, and explained colour vision in terms of three-coloured
receptors in the eye. Another supporter of the wave theory was
Leonhard Euler. He argued in
Nova theoria lucis et colorum (1746) that
diffraction could more easily be explained by a wave theory. In 1816
André-Marie Ampère gave
Augustin-Jean Fresnel an idea that the polarization of light can be explained by the wave theory if light were a
transverse wave.
[34]
Later, Fresnel independently worked out his own wave theory of light, and presented it to the
Académie des Sciences in 1817.
Siméon Denis Poisson
added to Fresnel's mathematical work to produce a convincing argument
in favour of the wave theory, helping to overturn Newton's corpuscular
theory.
[dubious – discuss]
By the year 1821, Fresnel was able to show via mathematical methods
that polarisation could be explained by the wave theory of light and
only if light was entirely transverse, with no longitudinal vibration
whatsoever.
[citation needed]
The weakness of the wave theory was that light waves, like sound
waves, would need a medium for transmission. The existence of the
hypothetical substance
luminiferous aether proposed by Huygens in 1678 was cast into strong doubt in the late nineteenth century by the
Michelson–Morley experiment.
Newton's corpuscular theory implied that light would travel
faster in a denser medium, while the wave theory of Huygens and others
implied the opposite. At that time, the
speed of light
could not be measured accurately enough to decide which theory was
correct. The first to make a sufficiently accurate measurement was
Léon Foucault, in 1850.
[35]
His result supported the wave theory, and the classical particle theory
was finally abandoned, only to partly re-emerge in the 20th century.
Electromagnetic theory
In 1845,
Michael Faraday discovered that the plane of polarisation of linearly polarised light is rotated when the light rays travel along the
magnetic field direction in the presence of a transparent
dielectric, an effect now known as
Faraday rotation.
[36] This was the first evidence that light was related to
electromagnetism. In 1846 he speculated that light might be some form of disturbance propagating along magnetic field lines.
[36]
Faraday proposed in 1847 that light was a high-frequency
electromagnetic vibration, which could propagate even in the absence of a
medium such as the ether.
[citation needed]
Faraday's work inspired
James Clerk Maxwell
to study electromagnetic radiation and light. Maxwell discovered that
self-propagating electromagnetic waves would travel through space at a
constant speed, which happened to be equal to the previously measured
speed of light. From this, Maxwell concluded that light was a form of
electromagnetic radiation: he first stated this result in 1862 in
On Physical Lines of Force. In 1873, he published
A Treatise on Electricity and Magnetism, which contained a full mathematical description of the behaviour of electric and magnetic fields, still known as
Maxwell's equations. Soon after,
Heinrich Hertz
confirmed Maxwell's theory experimentally by generating and detecting
radio waves in the laboratory, and demonstrating that these waves
behaved exactly like visible light, exhibiting properties such as
reflection, refraction, diffraction, and interference. Maxwell's theory
and Hertz's experiments led directly to the development of modern radio,
radar, television, electromagnetic imaging, and wireless
communications.
In the quantum theory, photons are seen as
wave packets
of the waves described in the classical theory of Maxwell. The quantum
theory was needed to explain effects even with visual light that
Maxwell's classical theory could not (such as
spectral lines).
Quantum theory
In 1900
Max Planck, attempting to explain
black-body radiation
suggested that although light was a wave, these waves could gain or
lose energy only in finite amounts related to their frequency. Planck
called these "lumps" of light energy "quanta" (from a Latin word for
"how much"). In 1905, Albert Einstein used the idea of light quanta to
explain the
photoelectric effect, and suggested that these light quanta had a "real" existence. In 1923
Arthur Holly Compton showed that the wavelength shift seen when low intensity X-rays scattered from electrons (so called
Compton scattering) could be explained by a particle-theory of X-rays, but not a wave theory. In 1926
Gilbert N. Lewis named these light quanta particles
photons.
[37]
Eventually the modern theory of
quantum mechanics came to picture light as (in some sense)
both a particle and a wave, and (in another sense), as a phenomenon which is
neither
a particle nor a wave (which actually are macroscopic phenomena, such
as baseballs or ocean waves). Instead, modern physics sees light as
something that can be described sometimes with mathematics appropriate
to one type of macroscopic metaphor (particles), and sometimes another
macroscopic metaphor (water waves), but is actually something that
cannot be fully imagined. As in the case for radio waves and the X-rays
involved in Compton scattering, physicists have noted that
electromagnetic radiation tends to behave more like a classical wave at
lower frequencies, but more like a classical particle at higher
frequencies, but never completely loses all qualities of one or the
other. Visible light, which occupies a middle ground in frequency, can
easily be shown in experiments to be describable using either a wave or
particle model, or sometimes both.
In February 2018, scientists reported, for the first time, the discovery of a new form of light, which may involve
polaritons, that could be useful in the development of
quantum computers.
[38][39]
See also